Download Clinical Lab Measures (Blood) Data Dictionary
Download Clinical Lab Measures (Urine) Data Dictionary
Download Research Lab Biomarkers and Genetic Data Dictionary
Biomarker and laboratory measures began at Visit 1 and were included at each subsequent research clinic visit, except Visit 11 and Visit 12. Clinical laboratory measures were conducted in various clinical laboratories using standardized methods for the relevant time period. Whole blood samples from Visit 5 were analyzed for APOE and VDR genotypes. Methodology and references for each group of variables are provided following the summary table below. Details of individual markers under each category are available below at Laboratory Sites and Methods. The term “Biomarker” refers to bio-measures that were conducted in research laboratories using methods that often represented the “gold standards” for those measures at that time.
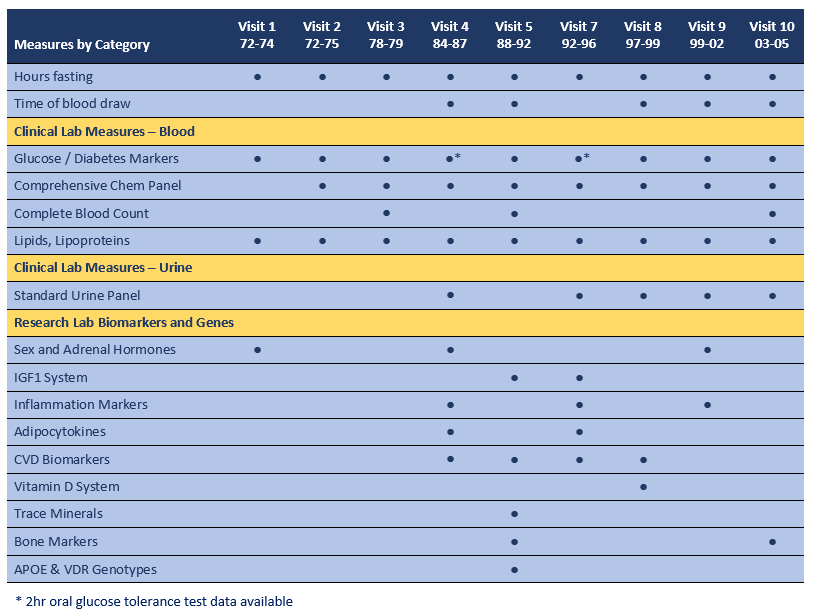
“Hours fasting” is available for all visits and was calculated as hours since reported last caloric intake and time of blood draw, as recorded by the research clinic nurse. For all visits, with the exception of Visit 5, participants were requested to fast for a minimum of 12 hours prior to arriving for their clinic appointment. Visit 5 samples were collected irrespective of fasting status, although time of blood draw and hours fasting were recorded, and a subset of 485 participants were requested to fast. Although time of blood draw was recorded at all visits, data were not entered at Visit 1, Visit 2, Visit 3 and Visit 7.
Clinical Lab Measures from serum and plasma are available for Visits 1 thru 10, with multiple repeat measurements for most analytes. Measures in urine are available for Visit 4 and Visit 7 thru 10. Assays were conducted in a variety of commercial and institutional clinical laboratories using standard methods for the time period of the Visit. See below for details.
Research Lab Biomarkers were targeted to specific research goals and were often measured at only one or two clinic visits. See below for information on laboratory, assay method and performance parameters for specific measures.
Laboratory Sites and Methods
This section provides methods text and references for clinical lab measures, biomarkers and genotypes. References are publications by Rancho Bernardo Study investigators. Additional references and details may be available in the publications cited for each method, as well as comments on the strengths and/or limitations of the methods used (see Discussion sections in each publication).
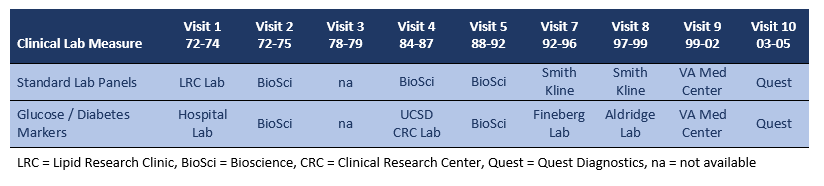
CLINICAL LAB MEASURES are available for Visits 1 thru 10, with repeat measurements for most markers. Assays were conducted in a variety of commercial and institutional clinical laboratories using standard methods for the time period of the visit. The table below lists the laboratory sites for each visit.
Glucose and Diabetes Markers were part of the clinical lab metabolic panel for all visits with the exception of Visit 1, Visit 4, Visit 7 and Visit 8. At Visit 4 and Visit 7, 2hr oral glucose tolerance tests were administered. See below for details on markers measured at these visits.
Visit 1: Fasting glucose was measured in a hospital diagnostic lab using the hexokinase method, which measures true glucose. (1)
Visit 4: A 75-g, 2hr oral glucose tolerance test was administered in the early morning hours. Assays were conducted at the UCSD Clinical Research Center (CRC) laboratory. Fasting and post-challenge plasma glucose levels were measured by the glucose oxidase method. Fasting and 2hr post-challenge plasma insulin levels were determined by double-antibody radioimmunoassay. (2)
Visit 7: A 75-g, 2hr oral glucose tolerance test was administered in the early morning hours. Assays were conducted at the research laboratory of S. Edward Fineberg (Indiana University). Fasting and 2hr post-challenge insulin levels were measured with a human insulin-specific double-antibody RIA kit (Linco Research, St. Louis, MO); the sensitivity was 2 μU/mL. Two levels of control external standards were used. Intra-assay and inter-assay coefficients of variations (CV) for control level 1 were 6% and 15%, respectively, and for control level 2, 4% and 5%, respectively. Proinsulin was measured with a rapid and sensitive RIA. Human insulin and C-peptide do not cross-react with proinsulin in this assay. Sensitivity was 2 pmol/L, and intra-assay and inter-assay CV were 5% to 16%. C-peptide was measured with an RIA assay kit (Linco Research, St. Louis, MO) with a sensitivity of 0.10 ng/mL. External controls used Biorad levels 1 through 3, with intra-assay CV of 3% to 5% and inter-assay CV of 11% to 18%. Cross-reactivity between proinsulin and C-peptide was <4%. (3)
Visit 8: Fasting glucose and insulin levels were measured at the Aldridge laboratory, University of Washington. Glucose was measured by the glucose oxidase method, insulin by double-antibody radioimmunoassay.
Glycosylated hemoglobin A1C
Visit 3: Glycosylated hemoglobin was determined as the total HbA1 fraction by an ion-exchange chromatographic method using the QUIK-SEP Fast Hemoglobin Test (Isolab incorporated, Akron, OH).(4).
Visit 4: HbA1C was measured at Visit 4 with high-performance liquid chromatography (GHBHPLC) or column chromatography (GHB_COL) using an automated analyzer (normal range 4.5–6.5%) (SmithKline, Van Nuys, CA) (5).
Visit 7: Hemoglobin A1C was measured by 4 methods (SmithKline, Van Nuys, CA). Each participant’s samples were measured by only one method depending on the timing of their clinic visit; variable names reflect the method used as follows. The original test, Method 1 or hemoglobin A1C by HPLC (GHBHPLC) and was used for 1480 samples. For 32 participants, Method 1 could not be used because of interference by a hemoglobin variant in the sample. In these cases, an alternative test was used, hemoglobin by affinity chromatography (GHBAFF). Effective July 10, 1995, a new method was used, called “glycohemoglobin A1C” (GHBA1C), which replaced the former hemoglobin A1C by HPLC (GHBHPLC). This method was used for 100 samples. In December 1995, a fourth method appeared, called “Hemoglobin A1C by IEC (International Expert Committee)” (GHBIEC). This method was used for 182 samples.
Visit 8: Hemoglobin A1C was measured at SmithKline laboratories by HPLC (GHBHPLC3).
Visit 9: Hemoglobin A1C was measured at the VA Medical Center in San Diego using the BIORAD variant HPLC method (GHB_BRV).
The comparability of the various methods used to measure HBA1C is unknown to the RBS archiving team.
Lipids and Lipoproteins for Visit 1 were measured in the core lipid laboratory for the Lipid Research Clinic Program using an AAI auto-analyzer. (6) Lipids for Visit 3, Visit 4 and Visit 7 were measured in a certified Lipid Research Clinic laboratory at the University of California San Diego.
Visit 1: Fasting plasma cholesterol and triglyceride levels were determined using an Auto-analyzer (Technicon Instruments Corp., Tarrytown, NY).
Visit 4 and Visit 7: Total cholesterol and triglyceride levels were measured by enzymatic techniques using an ABA-200 biochromatic analyzer (Abbott Laboratories, Irving, Texas). High-density lipoprotein (HDL) cholesterol was measured by precipitating other circulating lipoproteins with heparin and manganese chloride according to the standard Lipid Research Clinics protocol. Low-density lipoprotein (LDL) cholesterol was estimated using the Friedewald formula. (7)
For all other research clinic visits, lipids and lipoproteins were measured in a clinical laboratory as part of a standard metabolic panel.
Serum Albumin and Creatinine were part of a clinical panel except for Visit 7 and Visit 10.
Visit 7: Serum creatinine was measured using a variation of the Jaffe enzymatic method at a SmithKline Beacham laboratory on a Hitachi 911 analyzer (Roche Diagnostics, Indianapolis, IN), with inter- and intra-assay CVs of 4.0%.
Visit 10: Serum creatinine was measured in the commercial Quest laboratory using a modified Jaffe enzymatic method on an Olympus 5400 (Olympus Diagnostic Systems and Beckman Coulter; Brea, CA). (8)
URINE LAB MEASURES. Most urine measures were assayed in the clinical laboratory at UCSD using standard urine panels. Urine albumin and creatinine measures were measured at an NIH laboratory, as described below. At Visit 4 and Visit 7, both UCSD clinical lab and NIH lab measures of urine creatinine are available.
Visit 4, 7, 8, 9 and 10: Urine albumin was measured on clean-catch, untimed morning urine samples at the NIH laboratory (Phoenix, AZ) of Dr. Peter Bennett using the Behring Nephelometer BNA (Dade Behring GmbH, Marburg, Germany). The lower limit of detection was 6.8 mg/dl; interassay CV was 4.5%. Urine creatinine was measured in the same lab by the kinetic alkaline picrate method using the Ciba-Corning Express (Corning, Medfield, MA). (9)
RESEARCH LAB BIOMARKERS AND GENOTYPES were targeted to specific research goals and were often measured at only one or two research clinic visits. See below for information on laboratory, assay methods and quality control measures for specific analytes.
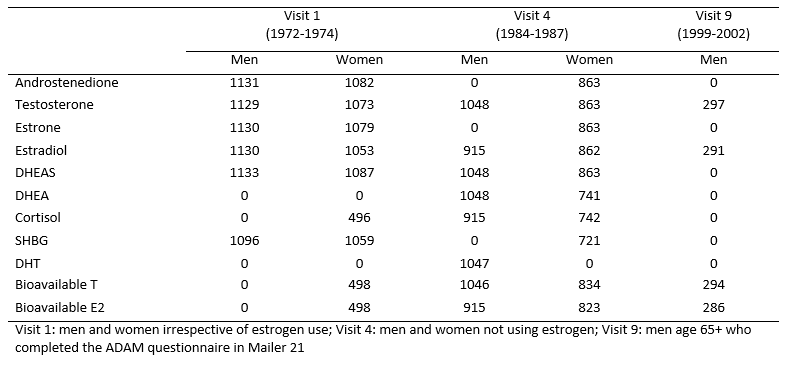
Sex and Adrenal Hormones for Visit 1, Visit 4 and Visit 9 were measured in the Reproductive Endocrinology Research laboratory of Samuel S.C. Yen at UCSD. The same methods were used for each visit; however, standards for some measures changed over the 9 to 14 year assay interval, thus longitudinal comparisons of absolute sex and adrenal hormone levels are not recommended. Plasma (Visit 1) and serum (Visit 4 and Visit 9) were obtained by venipuncture between 0730 and 1100 h from fasting participants and stored frozen at -70 degrees until assay. Sex hormone and DHEA levels were measured by RIA after solvent extraction and celite column chromatography; procedural losses were monitored by addition of tritiated standard to each sample before the extraction step. SHBG levels were determined by the charcoal-dextran precipitation method of Rosner. Bioavailable estradiol (BioE2) and bioavailable testosterone (BioT) (the non-SHBG-bound fractions) were measured by a modification of the ammonium sulfate precipitation technique of Tremblay and Dube. DHEAS, cortisol levels and DHT were measured by direct radioimmunoassay.
Visit 1: Assays were performed on first thawed plasma samples between 1985 and 1986 on a subset of participants selected randomly on the basis of sample availability, without knowledge of sex, age or estrogen use status. The sensitivity and intra- and inter-assay coefficients of variation, respectively, were 26 pmol/L (7 pg/ml), 15% and 16% for estrone; 18 pmol/L (5 pg/ml), 8% and 12% for estradiol; 4 pmol/L (1 pg/ml) x percentage bioavailable, 4% and 4% for bioavailable estradiol; 0.10 nmol/L (25 pg/ml), 4% and 10% for testosterone; 0.03 nmol/L (8 pg/ml), 6% and 6% for bioavailable testosterone; 1.8 nmol/L (30 pg/ml), 4% and 8% for androstenedione; 0.05 μmol/L (20 μg/L), 5% and 10% for DHEAS; and 2.6 nmol/L (9 ng/ml), 5% and 7% for cortisol. (10, 11)
Visit 4: Assays were performed on first thawed serum samples between 1992 and 1994; only men and non-estrogen using women were assayed. The sensitivity and the intra- and inter-assay coefficients of variation, respectively, were 11 pmol/L (3pg/ml), 6.0%, and 8% for estrone; 11 pmol/L, (3 pg/ml) 6%, and 7% for estradiol; 11 pmol/L (3 pg/ml) x percentage bioavailable, 6%, and 8% for bioavailable estradiol; 0.07 nmol/L (20 pg/ml), 4%, and 5% for testosterone; 0.07 nmol/L (20 pg/ml) x percentage bioavailable, 7%, and 11% for bioavailable testosterone; 0.06 nmol/L (17 pg/ml), 4%, and 4% for androstenedione; 0.14 nmol/L (0.04 ng/ml), 6.1% and 7.1% for DHEA; 0.22 μmol/L (80 μg/L), 3.1% and 7.3% for DHEAS; and 4 nmol/L (15 ng/ml), 5.4% and 10.5% for cortisol, and 5 nmol/L, 8%, and 11% for SHBG. (12, 13) Dihydrotestosterone (DHT) levels were measured in men only by RIA using the testosterone antibody after removing testosterone on a micro-celite column. The DHT assay sensitivity was 0.12 nmol/L (35 pg/ml), intra- and inter-assay coefficients were 7.5% and 7.5%. (14)
Visit 9: Total and bioavailable estradiol and testosterone measurements were conducted in 2007 on serum samples from 243 men ages 65 to 97 who attended Visit 9 (1999-2002) and completed the Androgen Deficiency in the Aging Male (ADAM) questionnaire in Mailer 21 (2002). Assay performance for Visit 9 was similar to that for Visit 4; specific details are not available.
Number of Participants with Sex and Adrenal Hormone Measurements by Visit and Sex
IGF System components were measured on Visit 5 blood samples obtained by venipuncture between 0800 and 1300 h; serum was separated and frozen at −70 C. Subjects were not required to be fasting when blood was collected; however, the exact time of blood draw and the time of last food or drink consumption were recorded. Serum IGF-I and IGFBP-1 levels were measured on twice-thawed samples between 2000 and 2001 in the Maine Center for Osteoporosis Research and Education Laboratory under the direction of Dr. Clifford J. Rosen. Serum IGF-I was determined by RIA using a commercial kit (Nichols Institute Diagnostics, San Clemente, CA) modified to optimize sensitivity and specificity; samples were diluted 1:105 (instead of 1:225) and were pretreated by acid-ethanol cryoprecipitation to remove IGFBPs. The assay sensitivity was 6.3 ng/ml; intra- and inter- assay coefficients of variation were 3% and 11%, respectively. IGFBP-1 levels were measured by an immunoradiometric assay kit (Diagnostics Systems Laboratories, Inc., Webster, TX) with a sensitivity of 0.33 ng/ml and intra- and inter-assay coefficients of variation of 4% and 14%, respectively. (15) IGF-1 levels were also measured at Visit 7 as part of a clinical lab panel; samples were drawn fasting between 0700 and 1130h; see Clinical Lab Measures – Blood for access to these IGF-1 data.
Inflammation Markers
Visit 4: Plasma interleukin-6 (IL-6) and high-sensitivity C-reactive protein (hsCRP) levels were measured on first-thawed Visit 4 samples in 2000. IL-6 was measured using a high-sensitivity amplified commercial ELISA with an alkaline phosphatase signal amplification system (R&D Systems, Minneapolis, MN). The intra- and interassay coefficients of variation for IL-6 ranged from 7–12% and from 8–13%, respectively; sensitivity was 0.094 pg/ml. Duplicate measurements of IL-6 were performed; the mean of the measurements was archived. High-sensitivity CRP was measured with an automated, high-sensitivity immunonephelometry method (Dade Behring, Inc., Deerfield, IL) with a sensitivity of 0.2 mg/L. The intra-assay coefficients of variation for standard concentrations at 15, 25, and 60 mg/L are 4.0, 2.4, and 4.4% (n = 20), respectively. The inter-assay coefficients of variation for standards at various concentrations range from 2.6–5.7%. (16)
Visit 7: Inflammatory markers were measured in 2010 on Visit 7 fasting EDTA plasma samples for the subset of participants in the Coronary Artery Calcification (CAC) study (see Cardiovascular Disease data group). Measurements for interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were conducted in the USC research laboratory of Frank Stancyzk using sensitive ELISA (R&D Systems, Minneapolis, MN) with intra- and inter-assay CVs of 5% and 10%, respectively, for IL-6, and 6% and 10%, respectively, for TNF-α. (17)
Visit 9: CRP levels were also measured at Visit 7 as part of a clinical lab panel; samples were drawn fasting between 0700 and 1130h; see Clinical Lab Measures – Blood for access to these data.
Adipocytokines
Visit 4: Adiponectin, ghrelin and leptin levels were measured on the same Visit 4 serum samples as the sex hormones, that is, in men and non-estrogen using women only. Measurements were conducted in 2004 by RIA at Linco Diagnostics Laboratory (St. Louis, MO). Linco reported no problems with two freeze‐thaw cycles for these assays. The Linco adiponectin assay measures total adiponectin, that is, all molecular forms. The sensitivity and the intra‐ and inter-assay coefficients of variation, respectively, were 0.8 mg/L, 6% and 7% for adiponectin; 95 pg/ml, 8% and 15% for ghrelin, and 0.5 ng/ml, 4% and 5% for leptin. (18)
Visit 7: Adiponectin and leptin levels were measured in 2010 on Visit 7 fasting EDTA plasma samples for the subset of participants in the Coronary Artery Calcification (CAC) study (see Cardiovascular Disease data group). Measurements were conducted in the USC research laboratory of Frank Stancyzk using radioimmunoassay kits (Millipore Linco Research, St Charles, MO) with intra- and inter-assay coefficients of variation (CVs) ranging from 2% – 7% for adiponectin and 4% – 6% for leptin. (17)
Cardiovascular Markers
Lp-PLA2 mass levels were measured in 2005 using the PLAC test (diaDexus, Inc., South San Francisco, CA) on Visit 4 sera stored frozen and thawed two times previously. The assay was a microplate-based ELISA, employing two monoclonal antibodies specific for Lp-PLA2, and calibrated to a recombinant enzyme standard. The assay sensitivity was 1.3 ng/ml and intra- and inter-assay coefficients of variation were <6% and <9%, respectively. The manufacturer reports that samples can be frozen and thawed up to six times without affecting Lp-PLA2 quantification. (7)
OPG & RANKL (osteoprotegerin and receptor activator of nuclear factor kappa-Β ligand, respectively) were measured on serum samples obtained at Visit 5 and Visit 7 (Coronary Artery Calcification (CAC) subset) and stored frozen at −70 C. Assays were conducted in 2004 at Amgen, Inc. (Thousand Oaks, CA) using commercial ELISA kits (Biomedica Gruppe, Vienna, Austria). Serum OPG was measured using an enzyme-linked immunosorbent assay (ELISA) kit that detects monomeric, dimeric, and ligand bound OPG. The published sensitivity of the assay was 2.8 pg/ml; the intra- and interassay coefficients of variation were 4–10% and 7–8%, respectively. RANKL levels were assayed using an ELISA that detects soluble, uncomplexed human RANKL in serum. The manufacturer’s insert indicates that the ELISA can reliably detect values below 1.6 pg/ml using extrapolation. The intra- and interassay coefficients of variation for RANKL were 3–5% and 6–9%, respectively. (19, 20)
Endothelin-1 levels were measured in serum samples collected during Visit 8 and frozen at -70°C. Assays were conducted in 2002 in the laboratory of Sidney Shaw in Bern, Switzerland. Endothelin-1 levels were measured using radioimmunoassay methods optimizing antibody-binding parameters; intra- and inter-assay coefficients of variation were 9% and 14%, respectively. (21)
Fetuin-A levels were measured in duplicate in EDTA plasma samples collected at Visit 7. Assays were conducted in 2010 using a human enzyme linked immunosorbent assay kit (Epitope Diagnostics, San Diego, CA). This assay uses a 2-site “sandwich” technique with polyclonal antibodies that bind different epitopes of human fetuin-A. Intra- and inter-assay coefficients of variation (CV) were 2.4–4.7% and 9.5–9.9%, respectively, for the set of assays used for the present sample.(22)
Vitamin D System: 25(OH)D, 1,25 (OH)2D and PTH were measured on fasting serum samples collected at Visit 8 and stored in tubes protected from sunlight. Measurements were conducted at the Holick-Chen laboratory at Boston University (Boston, MA). Serum 25(OH)D was measured using a vitamin D competitive protein binding protein assay and chemiluminescence detection; intra- and inter-assay coefficients of variation were 8% and 10%, respectively. The limit of detection was 5.0 ng/mL and the reference range was 10–55 ng/mL. Serum 1,25(OH)2D was measured using a radioreceptor assay; intra- and inter-assay coefficients of variation were 10% and 15%, respectively, with a reference range of 20–45 ng/L. PTH was measured using a chemiluminescence assay using kits from Nichols Institute Diagnostics (San Clemente, CA) for the measurement of intact PTH; intra- and inter-assay coefficients of variation were both 6% with a reference range of 10–65 ng/L. (23)
Trace Minerals were measured in Visit 5 plasma samples at the Grand Forks Human Nutrition Research Center in North Dakota. The samples were diluted 1:4 with distilled deionized water before analysis. Serachem Clinical Chemistry Control Serum Concentration 1 (Instrumentation Laboratory Co., Lexington, MA) and UTAK Normal Range Control Serum (UTAK Laboratories, Valencia, CA), prepared the same as the plasma samples, were used for quality assurance/quality control. The inductively coupled plasma (ICP) atomic emission spectrometer (Thermo Optek Corp., Franklin, MA) was used for rapid, simultaneous assay of plasma samples for iron, copper, and zinc. Results and performance were essentially the same as those using the atomic absorption spectrometer (AAS, Perkin-Elmer Corporation, Norwalk, CT), which measures only one element at a time. Comparisons of assay values from 20 random samples with both techniques indicated less than 3–5% differences between the ICP and AAS results, which approximates the expected day-to-day variability in the analyses (D. Milne, personal communication, May 5, 1993). (24)
Bone Markers
Visit 5: N-telopeptide (NTX) levels were measured in 1996 in morning fasting urine samples from Visit 5 using an enzyme-linked immunosorbent assay (ELISA) (Ostex International, Seattle, WA). For normalization of urinary NTX, urine creatinine was measured by an enzymatic assay (Ostex International). The assay was calibrated using standard amounts of human bone collagen digested with bacterial collagenase, and the results were expressed as nanomoles of bone collagen equivalents per millimoles of creatinine (nmol BCE/mmol CR). (25)
Visit 10: Intact procollagen type 1 n-terminal propeptide (P1NP) levels were measured in fasting serum samples in 2008 at Orion Diagnostica Oy (Finland) using the Orion Diagnostica UniQ assay. The inter-assay CV for the range of 40-134 μg/l was 4.5-7.4% based on 3 serum controls. N-telopeptide (NTX) levels were measured in fasting serum and in spot urine samples from Visit 10 participants. Assays were conducted at SPD Development Company Limited (Bedford, UK) using the Osteomark ELISA (Unipath Ltd, UK); intra- and inter- assay coefficients of variation ranged from 2–6%. (26) Urine NTX levels were also measured on the same samples at the same time using the VITROS assay.
OPG & RANKL (osteoprotegerin and receptor activator of nuclear factor kappa-Β ligand, respectively) were measured on serum samples obtained at Visit 5 and Visit 7 (Coronary Artery Calcification (CAC) subset) and stored frozen at −70 C. Assays were conducted in 2004 at Amgen, Inc. (Thousand Oaks, CA) using commercial ELISA kits (Biomedica Gruppe, Vienna, Austria). Serum OPG was measured using a kit that detects monomeric, dimeric, and ligand bound OPG. The published sensitivity of the assay was 2.8 pg/ml; the intra- and inter-assay coefficients of variation were 4–10% and 7–8%, respectively. RANKL levels were assayed using an ELISA that detects soluble, uncomplexed human RANKL in serum. The manufacturer’s insert indicates that the ELISA can reliably detect values below 1.6 pg/ml using extrapolation. The intra- and inter-assay coefficients of variation for RANKL were 3–5% and 6–9%, respectively. (19, 20)
Genotypes
DNA for genotyping was extracted from whole blood samples collected at Visit 5 and stored frozen at -70°.
APOE genotype: DNA was extracted by Sequana Therapeutics (La Jolla, CA) using standard techniques (PUREGENE). APOE genotype was determined by gel electrophoresis following polymerase chain reaction amplification around the diagnostic polymorphic sites. (27) See Siest and colleagues (Clinical Chemistry, 41, 1068–1086, 1995) for additional details regarding the APOE genotyping. Data are archived by numerical code for e2e2, e2e3, e2e4, e3e3, e3e4, and e4e4 genotypes.
Vitamin D Receptor (VDR) genotype: DNA was extracted using the Qiagen (Valencia, CA) blood DNA extraction kit. Primers were used to amplify a 900-bp fragment of DNA using the polymerase chain reaction (PCR) technique to determine the BsmI genotype. The fragment was subsequently digested using the restriction endonuclease BsmI. The ApaI and Taq I genotypes were determined by digestion of a 280-bp fragment amplified using the primers 5’GGTGGGATTGAGCAGTG39 and 5’ATGCTGCACTCAGGCTG39. In each case, PCR was performed using 20 ng of genomic DNA in a final reaction volume of 20 mL, and amplification occurred during 35 cycles of 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 30 seconds. Of the PCR product, 15 mL was incubated with 7.5 U of restriction endonuclease for 2 hours in a final volume of 20 mL. Digestion with TaqI (Stratagene, La Jolla, CA) and BsmI (Stratagene) occurred at 65°C and with ApaI (Stratagene) at 37°C. All products were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining under ultraviolet light (UV) light. The genotype of each sample was determined by 2 technicians working independently, and 10% of samples were genotyped at each site more than once to ensure that 100% reproducibility was achieved. (28)
VDR genotype data are contained in two variables for each of the 3 markers genotyped (TaqIs, ApaI, BSMI). The two variables represent two alleles at each locus (e.g. TaqI_a, TaqI_b). Each variable is coded so that lowercase letters denotes the presence and capital letters denote the absence of the site of the restriction enzymes Bsml (b/B), Apal (a/A), and Taql (t/T) on each of the alleles. (28)
REFERENCES
1. Barrett-Connor E, Khaw KT. Diabetes mellitus: an independent risk factor for stroke? Am J Epidemiol. 1988;128(1):116-23. PubMed PMID: 3381820.
2. Oh JY, Barrett-Connor E, Wedick NM, Wingard DL. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo Study. Diabetes Care. 2002;25(1):55-60. PubMed PMID: 11772901.
3. Oh JY, Barrett-Connor E, Wedick NM. Sex differences in the association between proinsulin and intact insulin with coronary heart disease in nondiabetic older adults: the Rancho Bernardo Study. Circulation. 2002;105(11):1311-6. PubMed PMID: 11901041.
4. Barrett-Connor E, Criqui MH, Witztum JL, Philippi T, Zettner A. Population-based study of glycosylated hemoglobin, lipids, and lipoproteins in nondiabetic adults. Arteriosclerosis. 1987;7(1):66-70. PubMed PMID: 3813976.
5. Kramer CK, Araneta MR, Barrett-Connor E. A1C and diabetes diagnosis: The Rancho Bernardo Study. Diabetes Care. 2010;33(1):101-3. doi: 10.2337/dc09-1366. PubMed PMID: 19837792; PMCID: PMC2797952.
6. Criqui MH, Barrett-Connor E, Holdbrook MJ, Austin M, Turner JD. Clustering of cardiovascular disease risk factors. Prev Med. 1980;9(4):525-33. PubMed PMID: 7403021.
7. Daniels LB, Laughlin GA, Sarno MJ, Bettencourt R, Wolfert RL, Barrett-Connor E. Lipoprotein-associated phospholipase A2 is an independent predictor of incident coronary heart disease in an apparently healthy older population: the Rancho Bernardo Study. J Am Coll Cardiol. 2008;51(9):913-9. doi: 10.1016/j.jacc.2007.10.048. PubMed PMID: 18308160; PMCID: PMC3096479.
8. Fung MM, Poddar S, Bettencourt R, Jassal SK, Barrett-Connor E. A cross-sectional and 10-year prospective study of postmenopausal estrogen therapy and blood pressure, renal function, and albuminuria: the Rancho Bernardo Study. Menopause. 2011;18(6):629-37. doi: 10.1097/gme.0b013e3181fca9c4. PubMed PMID: 21326121; PMCID: PMC3123422.
9. Jassal SK, Kritz-Silverstein D, Barrett-Connor E. A prospective study of albuminuria and cognitive function in older adults: The Rancho Bernardo Study. Am J Epidemiol. 2010;171(3):277-86, doi: 10.1093/aje/kwp426. PubMed PMID: 20061364; PMCID: PMC2842200.
10. Khaw KT, Barrett-Connor E. Lower endogenous androgens predict central adiposity in men. Ann Epidemiol. 1992;2(5):675-82. PubMed PMID: 1342319.
11. Tazuke S, Khaw KT, Barrett-Connor E. Exogenous estrogen and endogenous sex hormones. Medicine (Baltimore). 1992;71(1):44-51. PubMed PMID: 1549058.
12. Laughlin GA, Barrett-Connor E. Sexual dimorphism in the influence of advanced aging on adrenal hormone levels: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85(10):3561-8. doi: 10.1210/jcem.85.10.6861. PubMed PMID: 11061502.
13. Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Muhlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85(2):645-51. doi: 10.1210/jcem.85.2.6405. PubMed PMID: 10690870.
14. Trifiro MD, Parsons JK, Palazzi-Churas K, Bergstrom J, Lakin C, Barrett-Connor E. Serum sex hormones and the 20-year risk of lower urinary tract symptoms in community-dwelling older men. BJU Int. 2010;105(11):1554-9. doi: 10.1111/j.1464-410X.2009.09090.x. PubMed PMID: 20002438.
15. Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89(1):114-20. doi: 10.1210/jc.2003-030967. PubMed PMID: 14715837.
16. Wassel CL, Barrett-Connor E, Laughlin GA. Association of circulating C-reactive protein and interleukin-6 with longevity into the 80s and 90s: The Rancho Bernardo Study. J Clin Endocrinol Metab. 2010;95(10):4748-55. doi: 10.1210/jc.2010-0473. PubMed PMID: 20660034; PMCID: PMC3050106.
17. Wassel CL, Laughlin GA, Araneta MR, Kang E, Morgan CM, Barrett-Connor E, Allison MA. Associations of pericardial and intrathoracic fat with coronary calcium presence and progression in a multiethnic study. Obesity (Silver Spring). 2013;21(8):1704-12. doi: 10.1002/oby.20111. PubMed PMID: 23666866; PMCID: PMC3748173.
18. Langenberg C, Bergstrom J, Laughlin GA, Barrett-Connor E. Ghrelin, adiponectin, and leptin do not predict long-term changes in weight and body mass index in older adults: longitudinal analysis of the Rancho Bernardo cohort. Am J Epidemiol. 2005;162(12):1189-97. doi: 10.1093/aje/kwi338. PubMed PMID: 16236994.
19. Bakhireva LN, Laughlin GA, Bettencourt R, Barrett-Connor E. Does osteoprotegerin or receptor activator of nuclear factor-kappaB ligand mediate the association between bone and coronary artery calcification? J Clin Endocrinol Metab. 2008;93(5):2009-12. doi: 10.1210/jc.2007-2624. PubMed PMID: 18319315; PMCID: PMC2386279.
20. Stern A, Laughlin GA, Bergstrom J, Barrett-Connor E. The sex-specific association of serum osteoprotegerin and receptor activator of nuclear factor kappaB legend with bone mineral density in older adults: the Rancho Bernardo Study. Eur J Endocrinol. 2007;156(5):555-62. doi: 10.1530/eje-06-0753. PubMed PMID: 17468191; PMCID: PMC2642656.
21. Kanaya AM, Barrett-Connor E, Wassel Fyr CL. Endothelin-1 and prevalent coronary heart disease in older men and women (the Rancho Bernardo Study). Am J Cardiol. 2007;99(4):486-90. doi: 10.1016/j.amjcard.2006.09.096. PubMed PMID: 17293190; PMCID: PMC3947589.
22. Laughlin GA, Cummins KM, Wassel CL, Daniels LB, Ix JH. The association of fetuin-A with cardiovascular disease mortality in older community-dwelling adults: the Rancho Bernardo study. J Am Coll Cardiol. 2012;59(19):1688-96. doi: 10.1016/j.jacc.2012.01.038. PubMed PMID: 22554599; PMCID: PMC3345127.
23. Jassal SK, Chonchol M, von Muhlen D, Smits G, Barrett-Connor E. Vitamin d, parathyroid hormone, and cardiovascular mortality in older adults: the Rancho Bernardo Study. Am J Med. 2010;123(12):1114-20. doi: 10.1016/j.amjmed.2010.07.013. PubMed PMID: 20870200; PMCID: PMC3010282.
24. Lam PK, Kritz-Silverstein D, Barrett Connor E, Milne D, Nielsen F, Gamst A, Morton D, Wingard D. Plasma trace elements and cognitive function in older men and women: the Rancho Bernardo Study. J Nutr Health Aging. 2008;12(1):22-7. PubMed PMID: 18165841; PMCID: PMC2647138.
25. Schneider DL, Barrett-Connor EL. Urinary N-telopeptide levels discriminate normal, osteopenic, and osteoporotic bone mineral density. Arch Intern Med. 1997;157(11):1241-5. PubMed PMID: 9183236.
26. McDaniels-Davidson CR, Kritz-Silverstein D, Huang MH, Laughlin GA, Johnson S, Haapalahti J, Schneider DL, Barrett-Connor E, Kado DM. The association between bone turnover markers and kyphosis in community-dwelling older adults. Bone Rep. 2016;5:57-61. doi: 10.1016/j.bonr.2016.04.001. PubMed PMID: 27868084; PMCID: PMC4926834.
27. Petkus AJ, Wetherell JL, Stein MB, Liu L, Barrett-Connor E. History of sexual assault is associated with greater declines in executive functioning in older adults with APOE epsilon4. J Gerontol B Psychol Sci Soc Sci. 2012;67(6):653-9. doi: 10.1093/geronb/gbr163. PubMed PMID: 22357643; PMCID: PMC3478726.
28. Oh JY, Barrett-Connor E. Association between vitamin D receptor polymorphism and type 2 diabetes or metabolic syndrome in community-dwelling older adults: the Rancho Bernardo Study. Metabolism. 2002;51(3):356-9. PubMed PMID: 11887173.
